Figure 1. Work from the Liu group. Taken from Ref. [1].
The first 2 articles we are looking at this week are published as advance articles in Nature Chemistry [1, 3]. Both are very innovative work and there is a link between them. We will first take a look at the investigations and then look into the link – namely Photoredox catalysis.

Figure 2. The new reaction discovery by the Liu group. Taken from Ref. [1].
The first one is chemical biology carried out by Prof. David Liu’s group at Harvard (Figure 1,2). The innovative strategy of discovering novel chemical reactions blends chemistry and biology strategies (PCR) together [1]. They have developed a ‘DNA-templated reaction discovery system’ [1]. If the 2 reactants have a bond-formation process taken place (there is a reaction), an ‘in vitro selection, PCR Amplification and DNA microarray analysis’ will that specific combinations [1]. In this attempt they have succeeded in identifying the Ru (II)-catalyzed azide reduction protocol to amine, which was catalyzed by visible light. This methodology has the added advantage it is compatible at physiological conditions, which means that it can serve as a useful tool for synthesis concerning macromolecules.
The second one is carried out by Prof. Corey Stephenson’s group [3]. The conversion of an alcohol into an alkyl halide with triphenylphosphine is known as an Appel reaction. The mechanistic minds should immediately notice the inevitable formation of triphenylphosphine oxide – remember this fella from my article ‘Atom Economy’ back in June 2010? [2]
Stephenson’s group counteracted against this issue by using a Ruthenium salt and visible light as the catalyst (Figure 3)[3]. In the presence of the halide source (e.g. iodoform), the alkyl halide can be made with inversion of stereochemistry. But without the infamous phosphine oxide fella this time! This work is covered in an article of RSC Chemistry World [4].

Figure 3. Stephenson's Appel reaction. Taken from Ref.[3].
The common link is the photoredox catalysis involved, with the use of an interesting Ruthenium salt (Figure 3). In the presence of visible light, the Ru (II) salt is sensitized to a Ru(II)* state. With the further loss of an electron, Ru (III) is formed and the whole process is known as an oxidative quenching cycle. When Ru (III) is reduced back to Ru (II), this will enable the organic substrate to carry out an oxidation reaction, and continues further into the mechanistic sequences [1, 3]. The advantage of using visible light as a catalyst means that it can be developed as a green method of synthesis.
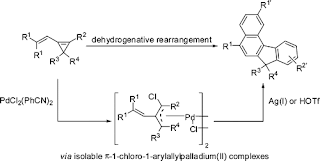
Indeed, this type of photocatalysis has been a hot area in synthetic chemistry. A recent JACS advance articles illustrates this notion. The reaction represents a cycloaddition of a linear precursor into a fused cyclopentane system, under photoredox catalysis and the presence of a Lewis Acid. It also illustrates the use of a cyclopropane as a 3-carbon building block (Figure 4) [5].

Figure 4. Yoon's cyclopentane synthesis. Taken from Ref. [5].
Prof. David MacMillan has also done impressive work in cascade organocatalytic work with the involvement of photoredox catalysis, employing both Ruthenium and Iridium salts (Figure 5)[6].

Figure 5. A nice example of MacMillan's organocatalytic synthesis. Taken from [6a].
Reference:
1. A biomolecule-compatible visible-light-induced azide reduction from a DNA-encoded reaction-discovery system
Yiyun Chen,Adam S. Kamlet,Jonathan B. Steinman& David R. Liu
Nature Chemistry 2011
doi:10.1038/nchem.932
2. For my article on ‘Atom Economy’ on 13/6/2010 see:
http://emockscience.blogspot.com/2010/06/c-6-carbons-12-carbons-18-carbons-waste.html
3.Visible-light-mediated conversion of alcohols to halides
Chunhui Dai,Jagan M. R. Narayanam& Corey R. J. Stephenson
Nature Chemistry 2011
doi:10.1038/nchem.949
4. The coverage in RSC Chemistry World:
http://www.rsc.org/chemistryworld/News/2011/January/09011101.asp
5. [3+2] Cycloadditions of Aryl Cyclopropyl Ketones by Visible Light Photocatalysis
Zhan Lu, Meihua Shen, and Tehshik P. Yoon
J. Am. Chem. Soc.
DOI: 10.1021/ja107849y
6. (a) Enantioselective α-Benzylation of Aldehydes via Photoredox Organocatalysis
Hui-Wen Shih, Mark N. Vander Wal, Rebecca L. Grange, and David W. C. MacMillan
J. Am. Chem. Soc., 2010, 132 (39), pp 13600–13603
DOI: 10.1021/ja106593m
(b) Enantioselective α-Trifluoromethylation of Aldehydes via Photoredox Organocatalysis
David A. Nagib, Mark E. Scott and David W. C. MacMillan
J. Am. Chem. Soc., 2009, 131 (31), pp 10875–10877
DOI: 10.1021/ja9053338
(c) Merging Photoredox Catalysis with Organocatalysis: The Direct Asymmetric Alkylation of Aldehydes
David A. Nicewicz and David W. C. MacMillan
Science 3 October 2008: 77-80
DOI:10.1126/science.1161976