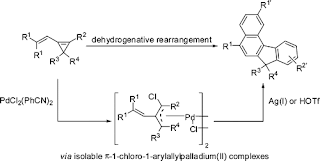
Figure 1. The dehydrogenative rearrangement to give aromatic systems by the Shi group. Taken from Ref. [1].
This article from Professor Shi’s group shows a number of innovations. Firstly, it reiterates the synthetic potential of cyclopropene, not only as a 3-carbon building block but also as an allylic disguise , when interacting with a metal such as palladium. Secondly, the simple precursor is capable of a dehydrogenative arrangement under palladium catalyst, forming benzene derivatives as a result, and lose H2 gas as a side-product. Dehydrogenative reactions involving transition metal catalysis is a hot area in current organic synthesis. When the structure of the starting precursor is complex, interesting polycyclic compounds form as a result.
Reference:
1. Preparation of Di-μ-chlorobis[π-1-chloro-1-aryl-2-(2′,2′-diarylvinyl)allyl]palladium(II) Complexes and a Novel Dehydrogenative Rearrangement of Arylvinylcyclopropenes for the Synthesis of 7H-Benzo[c]fluorene Derivatives
Zhi-Bin Zhu, Kai Chen, Yin Wei, and Min Shi
Organometallics, DOI: 10.1021/om1009846
No comments:
Post a Comment